On 15 December 2020, the European Commission has published Commission Regulation (EU) 2020/2096 to amend Annex XVII to REACH Regulation (EC) No 1907/2006. The amendments shall enter inforce on the twentieth day following that of its publication in the Official Journal of the European Union (5 January 2021) unless specified in individual substance. The following are the highlight of the amendments:

1.Amendments to Entry 3 (Liquid substances or mixtures which areregarded as dangerous)
1)The Risk Phase “R65” shall be deleted in this entry(point 3 and 5 of the restrictions).
2)The restrictions in point 6 and 7 shall be deleted.
2.The following Entries shall be deleted:
1)Entry 22 (Pentachlorophenol)
2)Entry 67 (Bis(pentabromophenyl)ether (decabromodiphenyl ether; decaBDE))
3)Entry 68 (Perfluorooctanoic acid (PFOA) and its salts) from 4 July 2020.
3.Amendments to Entry 46 ((a) Nonylphenol, (b)Nonylphenol ethoxylates)
The references to CAS number and EC number in paragraph (a) of column 1 are deleted.
4.Amendments to Entries 28, 29 and 30 (Substances which are classified as carcinogen category 1A or 1B, germ cell mutagen category 1A or 1Band reproductive toxicant category 1A or 1B)
The devices covered by Regulation (EU) 2017/745 shall not apply to the restrictions of these entries.
5.Amendments the title of Appendix 1~6
1)The title of Appendix 1 is replaced by the following:Entry 28 – Carcinogens: Category 1 A
2)The title of Appendix 2 is replaced by the following: Entry28 – Carcinogens: Category 1 B
3)The title of Appendix 3 is replaced by the following: Entry29 – Germ cell mutagens: Category 1 A
4)The title of Appendix 4 is replaced by the following: Entry29 – Germ cell mutagens: Category 1 B
5)The title of Appendix 2 is replaced by the following: Entry30 – Reproductive toxicants: Category 1 A
6)The title of Appendix 2 is replaced by the following: Entry30 – Reproductive toxicants: Category 1 B
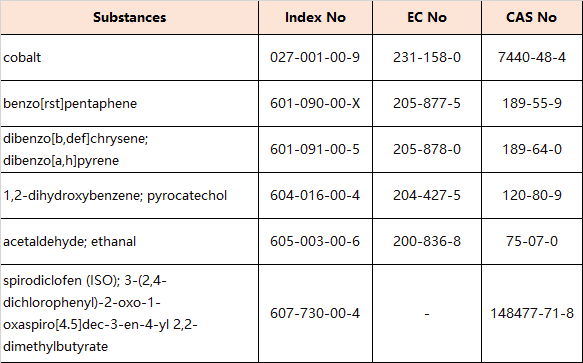
6. Amendments to Appendix 2 (Entry 28 – Carcinogens:Category 1B)
the following entries are inserted in the table in order of the index numbers set out therein:
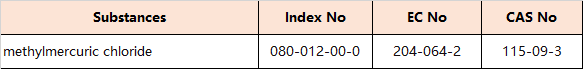
7.Amendments to Appendix 5 (Entry 30 – Reproductive toxicants: Category 1A)
the following entries are inserted in the table in order of the index numbers set out therein:
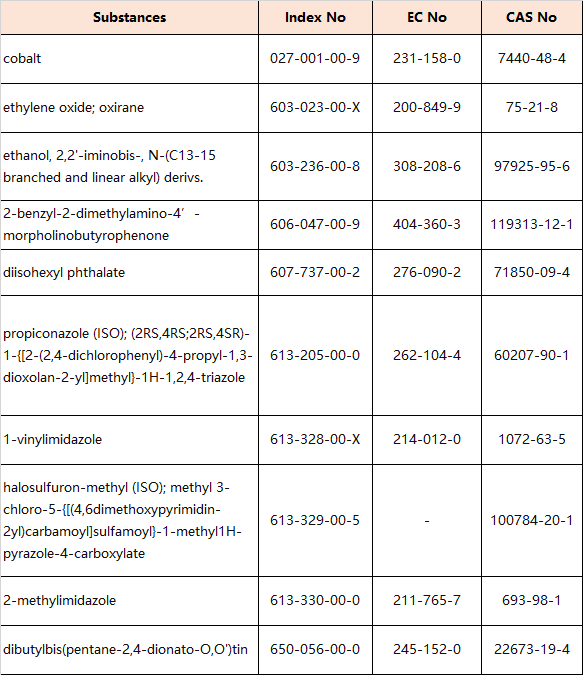
8.Amendments to Appendix 6 (Entry 30 – Reproductive toxicants: Category 1B)
the following entries are inserted in the table in order of the index numbers set out therein:
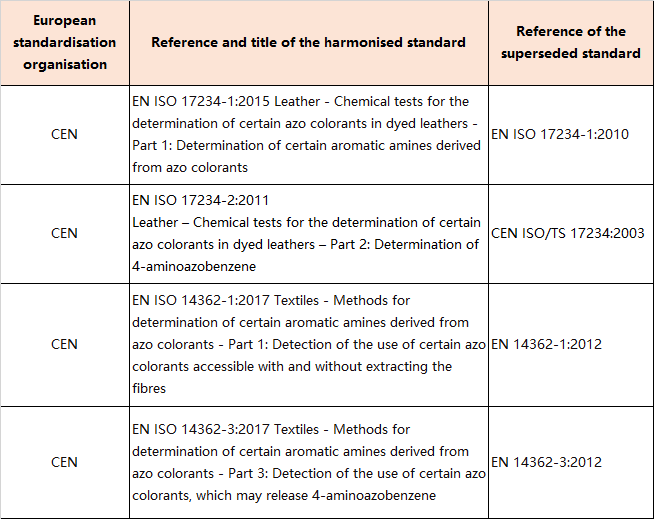
9.Amendments to Appendix 10 (Entry 43 - Azocolourants -List of testing methods)
In Appendix 10, the tableis replaced by the following table:
link: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020R2096&qid=1608184582666
